Study findings support the recent FDA approval of the immunotherapy agent for patients whose cancers have high number of mutations.
9:33 AM
Author |

The immunotherapy agent pembrolizumab can provide clinical benefit to some patients with metastatic breast cancer whose tumors were found to have a high number of mutations, and whose cancer continued to progress with standard treatments.
That's according to newly published results from ASCO's Targeted Agent and Profiling Utilization Registry (TAPUR) study. The findings appear in the Journal of Clinical Oncology.
The goal of TAPUR is to use next-generation genomic sequencing data to assess whether cancer drugs previously approved in one cancer type might benefit additional groups of patients across cancer types due to specific alterations to DNA and RNA in their tumors.
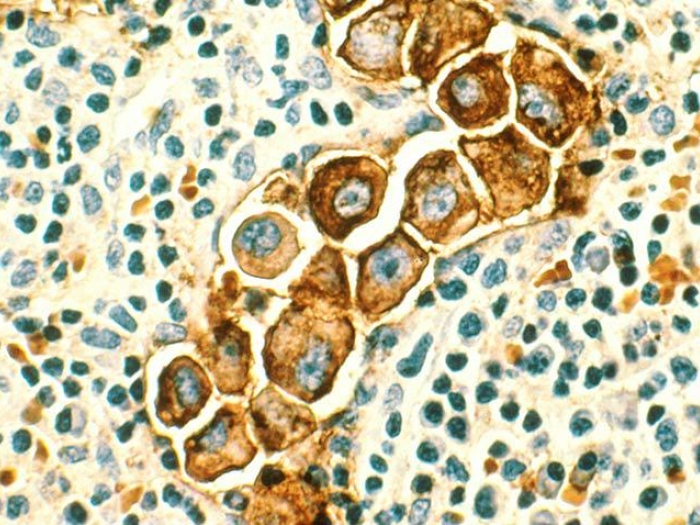
For this research, 28 women with advanced breast cancer were enrolled at multiple trial sites between 2016 and 2018. Each had advanced breast cancer with a large number of mutations in their tumors — what doctors call a "high tumor mutational burden" — and had already been through several rounds of treatment.
The phase 2 study showed that just over a third of the trial participants' cancers had their tumors controlled with pembrolizumab and a small number of patients saw benefits lasting a year or more.
Like Podcasts? Add the Michigan Medicine News Break on iTunes, Google Podcast or anywhere you listen to podcasts.
"Immunotherapy doesn't work for every patient, but when it does work, it can have really profound effects," says study lead author Ajjai Alva, MBBS, an oncologist at Michigan Medicine and principal investigator for the University of Michigan trial site. "When it comes to a biomarker to tell us which patients will respond best to immunotherapy, we still haven't found the perfect marker, the holy grail — but we have found a very good candidate biomarker in high tumor mutational burden."
Pembrolizumab is a type of immunotherapy known as a checkpoint inhibitor. By blocking the binding of a protein called PD-1, pembrolizumab releases the brakes on the patient's immune response, enabling the patient's body to better attack the cancer.
There's growing evidence that next-generation sequencing should be considered part of the standard of care for all patients with advanced cancers.Ajjai Alva, MBBS
"Mutational burden has been shown to be helpful in identifying patients likely to benefit from pembrolizumab across multiple cancer types. And our results in metastatic breast cancer are consistent to those reported since in other solid tumors," Alva says.
Last year, as the study was being completed, the federal Food and Drug Administration approved the use of pembrolizumab to treat patients with any type of unresectable or metastatic solid tumor if the cancer had a high mutational burden and continued to progress despite standard treatments.
"Our data provide additional support for that approval," says Alva, who presented initial results from the study at the American Society for Clinical Oncology's annual meeting in 2019. "Especially if you consider that data underlying that approval did not include any patients with breast cancer."
Moreover, Alva notes, the study is part of a larger movement toward individualized medicine through the use of next-generation sequencing and the molecular nuances it uncovers in each patient's tumor.
MORE FROM THE LAB: Subscribe to our weekly newsletter
Alva was also co-author of a recent U-M Rogel Cancer Center study that found nearly 40% of study participants with advanced cancer experienced some clinical benefit from sequencing, with 20% experiencing exceptionally good responses — defined as keeping their disease under control for at least one year.
"There's growing evidence that next-generation sequencing should be considered part of the standard of care for all patients with advanced cancers," he says.
Additional authors on the study include: Pam K. Mangat, Elizabeth Garrett-Mayer, Kaitlyn R. Antonelli, Nicole L. Butler, Sasha L. Warren, Andrew L. Rygiel, Shamika Ranasinghe, Suanna S. Bruinooge and Richard L. Schilsky of ASCO; Susan Halabi of Duke University; Damien Hansra, Eugene R. Ahn, Pamela Crilley and Sagun Shrestha of Cancer Treatment Centers of America; Carmen Julia Calfa of the Sylvester Comprehensive Cancer Center; Maged F. Khalil of the Lehigh Valley Cancer Institute; Timothy Lewis Cannon of the Inova Schar Cancer Institute; Julie Gottlieb Fisher of the Levine Cancer Institute; and Derrick S. Haslem of the Intermountain Logan Cancer Clinic.
The research was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Pembrolizumab was provided by Merck Sharp & Dohme Corp with additional funding provided by AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Genentech, and Pfizer.
Study cited: "Pembrolizumab in Patients with Metastatic Breast Cancer with High Tumor Mutational Burden: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study," Journal of Clinical Oncology. DOI: 10.1200/JCO.20.0292

Explore a variety of health care news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!