Tumors with alterations in the CDK12 gene were more responsive to immunotherapy, suggesting a precision medicine approach.
11:00 AM
Author |

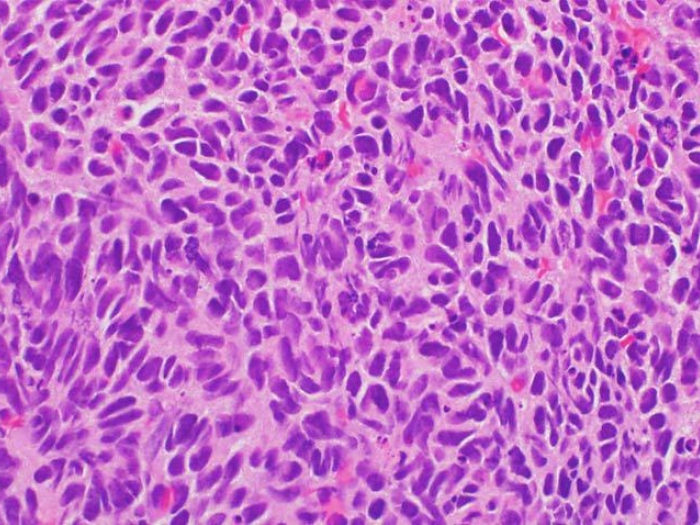
Researchers led by the University of Michigan Rogel Cancer Center have identified a new subtype of prostate cancer that occurs in about 7 percent of patients with advanced disease.
LISTEN UP: Add the new Michigan Medicine News Break to your Alexa-enabled device, or subscribe to our daily audio updates on iTunes, Google Play and Stitcher.
The subtype is characterized by loss of the gene CDK12. It was found to be more common in metastatic prostate cancer compared with early stage tumors that had not spread.
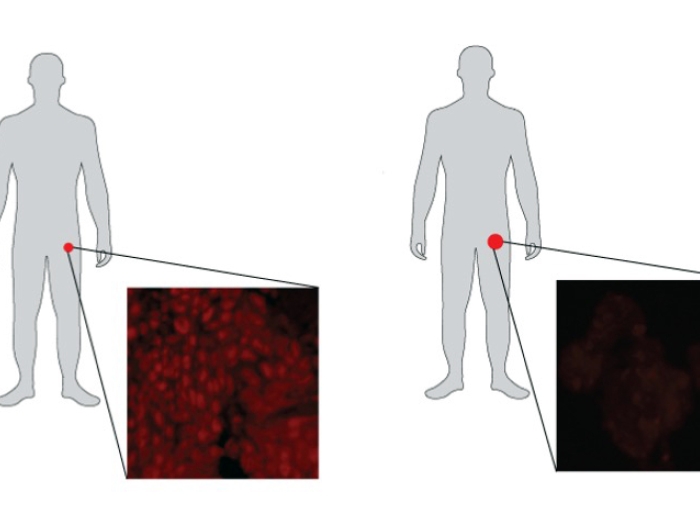
Tumors in which CDK12 was inactivated were responsive to immune checkpoint inhibitors, a type of immunotherapy treatment that overall has had limited success in prostate cancer.
"Because prostate cancer is so common, 7 percent is a significant number. The fact that immune checkpoint inhibitors may be effective against this subtype of prostate cancer makes it even more significant. This is an exciting prospect for patients who have CDK12 alterations and may benefit from immunotherapy," says senior study author Arul Chinnaiyan, M.D., Ph.D., director of the Michigan Center for Translational Pathology.
Researchers at the Rogel Cancer Center will lead a multisite clinical trial to assess checkpoint inhibitors as a treatment for metastatic prostate cancer with CDK12 loss.
In this study, published in Cell, researchers looked at DNA and RNA sequencing data from 360 tumor samples from patients with metastatic castration-resistant prostate cancer. This is an aggressive, advanced form of the disease in which the cancer has spread throughout the body and no longer responds to traditional hormone-based treatments. Tumor samples were from U-M's Mi-ONCOSEQ program and from samples collected through the Stand Up To Cancer-Prostate Cancer Foundation Dream Team.
MORE FROM THE LAB: Subscribe to our weekly newsletter
Researchers found loss of CDK12 in only about 1 percent of early prostate cancer samples. That jumped to 7 percent for metastatic cancer, which indicates a more aggressive form of the disease.
"It suggests that those early stage patients who have CDK12 loss are the ones who will develop metastatic disease. This could be a harbinger in early cancer," Chinnaiyan says.
By following the mechanism of how CDK12 loss impacts the cell, researchers found a process in which cells create neoantigens that are foreign to the immune system. This boosts immune-fighting T-cells, which may explain why these patients benefit from immune checkpoint blockade.
This is an exciting prospect for patients who have CDK12 alterations and may benefit from immunotherapy.Arul Chinnaiyan, M.D., Ph.D.
This suggests that a precision medicine approach to prostate cancer could help better direct immunotherapy treatment. It could also explain why some prostate cancer patients have had exceptional responses to immunotherapy while the treatment has had lackluster results overall in prostate cancer.
The team first recognized a possible role for CDK12 in a 2015 paper that evaluated the genomic landscape of advanced prostate cancers. CDK12 has also been linked to ovarian cancer.
Little is known about CDK12 on a molecular basis, but scientists do know that CDK12 regulates several critical cellular processes and is essential for development. Eliminating it is likely lethal to most cell types. So why can tumors lose CDK12 and survive? Researchers suspect cancer must inherit something that allows it to grow in the face of CDK12 loss. More study is needed to understand this.
"This very promising study suggests that CDK12 loss may be a biomarker for identifying prostate cancer patients who may respond to checkpoint immunotherapy," says Howard Soule, Ph.D., executive vice president and chief science officer of the Prostate Cancer Foundation.
"The Prostate Cancer Foundation is proud to have funded this team, which continues to make foundational strides in identifying actionable genomic mutations in prostate cancer and using this information to identify new classes of precision treatments that can be used to improve the lives of men with prostate cancer."
The University of Michigan has applied for a provisional patent and is presently seeking commercialization partners to help bring the technology to market.

Explore a variety of health care news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!