In aggressive prostate tumors that emerge after treatment, new laboratory studies find that inhibiting the protein LSD1 could lead to better outcomes
5:00 AM
Author |

A study from the University of Michigan Rogel Cancer Center uncovers a new mechanism to explain why some prostate tumors switch from a common, treatable form to a more rare and aggressive form of prostate cancer.
Using tissue samples and cell models from patients, Joshi Alumkal, M.D., Wicha Family Professor of Oncology and leader of the genitourinary medical oncology section at Rogel, and his team zeroed in on the lysine specific demethylase 1, a protein involved in turning genes off and on in normal and cancer cells that appears particularly important in certain aggressive forms of prostate cancer.
Further, they outlined a promising path to overcome this deadly form of treatment-resistance: LSD1 inhibitors.
The findings are published in JCI Insight.
Most prostate tumors remain adenocarcinomas, or glandular tumors, after male-hormone lowering treatments—the principal treatment for metastatic prostate cancers.
But many undergo a deadly switch called lineage plasticity, where the tumor shifts from a glandular tumor to one with a nerve, or brain-like, make-up.
Researchers have a limited understanding of how prostate tumors undergo lineage plasticity, but once it happens, few treatment options exist.
“Aggressive forms of prostate cancer are on the rise as a work-around to some of our newer, more potent hormonal treatments,” said Alumkal.
“Our prior work demonstrated that approximately 15-20% of patients whose tumors start growing despite newer hormonal treatments will lose the adenocarcinoma program and take on other identities, including one called neuroendocrine prostate cancer.”
Patients with neuroendocrine prostate cancer fare much worse than patients whose tumors remain adenocarcinomas, and there are currently limited treatment options for these patients.
“Our laboratory is focused on understanding how prostate tumors shift away from a glandular program and ways to block this lineage switch,” said Alumkal, who also co-leads the Translational and Clinical Research Program at Rogel.
A promising countermeasure
For the past decade, Alumkal’s laboratory has been studying LSD1. He first showed that LSD1 was important for survival of prostate adenocarcinoma tumors by turning on genes that are linked with stem cells, primitive cells that give rise to multiple cell types and that are difficult to eradicate.
Building on that, his team sought to determine whether LSD1 also plays a role in neuroendocrine prostate cancer.
The answer is yes.
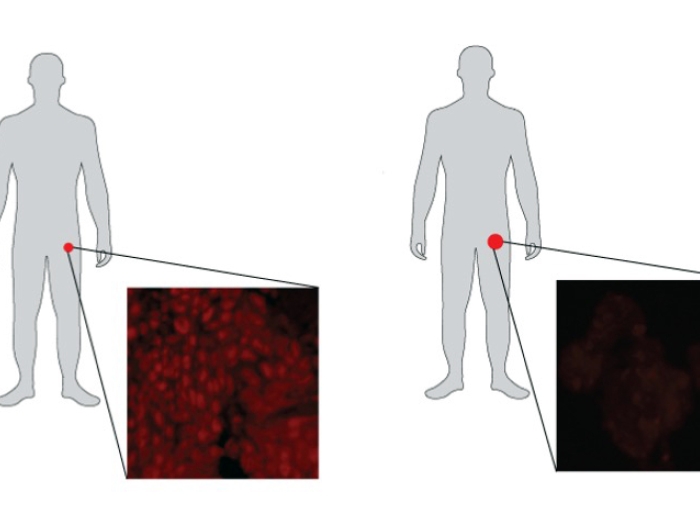
By examining tissues from patients with metastatic prostate cancer, the team determined that LSD1 was more highly expressed in neuroendocrine prostate tumors than in adenocarcinoma tumors.
They used a technique called RNA interference to remove LSD1 from neuroendocrine prostate cancer cells and found that the neuroendocrine prostate cancer models grew less well when LSD1 was absent, demonstrating LSD1’s importance for survival of these aggressive cells.
They then discovered that blocking LSD1’s interaction with other proteins was a more effective approach than blocking LSD1’s enzymatic function in neuroendocrine prostate cancer cells, consistent with their prior work in prostate adenocarcinoma tumors.
“Ultimately, we found that a class of drugs—allosteric inhibitors—that block protein-protein interactions was much more effective in stopping LSD1 and slowing the growth of cancer cells,” said Anbarasu Kumaraswamy, Ph.D., the first author and post-doctoral fellow in the Alumkal Laboratory.
Uncovering how LSD1 functions in neuroendocrine prostate tumors led the team to discover another dimension to this protein: LSD1 turns off p53, a tumor suppressor gene that acts as a brake in cancer cells.
When the team examined how genes changed after LSD1 was inhibited, reactivation of p53 came up again and again across all the cell models. The team confirmed that LSD1 was turning off p53, preventing it from binding to the DNA. LSD1 inhibitors reactivated p53 and suppressed tumor growth.
“That cell lines lacking p53 were less sensitive to LSD1 inhibition gives us strong clues about the importance of p53 reactivation for the anti-tumor effects of LSD1 inhibition,” Alumkal said.
The team tested LSD1 inhibitors in neuroendocrine prostate tumors implanted in mice. One of the drugs tested, seclidemstat, is in a phase 1 clinical trial in sarcoma.
In every case, seclidemstat blocked growth of the tumors, and in several tumors there was complete regression. Importantly, treatment was well-tolerated with no observed toxicities in the mice.
Alumkal says the research points toward LSD1 inhibition with this type of drug as potentially beneficial for patients with neuroendocrine prostate cancer.
“The fact that the drug we found is in clinical testing gives us hope that we might be able to develop clinical trials targeting LSD1 in aggressive prostate cancers in the near term,” he said.
“These findings could also lead to a more generalizable approach to reactivating p53 function in other cancers.”
Additional authors include:
- U-M Rogel Cancer Center: Anbarasu Kumaraswamy, Zhi Duan, Diana Flores, Chao Zhang, Olivia A. Swaim, Eva Rodansky, William K. Storck, Joshua A. Kuleape, Karan Bedi, Rahul Mannan, Xiao-Ming Wang, Aaron Udager, Visweswaran Ravikumar, Armand Bankhead III, Arul M. Chinnaiyan, Arvind Rao, Joel A. Yates, Joshi J. Alumkal
- Fred Hutchinson Cancer Center: Peter S. Nelson, Ilsa Coleman
- University of California Los Angeles: John K. Lee
- University of Washington: Colm Morrissey
- Oregon Health & Science University: Archana Sehrawat, Ya-Mei Hu, Zheng Xia
Funding includes: National Cancer Institute (R01 CA251245, P50 CA097186, P50 CA186786, NCI P50 CA186786-07S1, P30 CA046592, P30 CA015704, 5P01 CA163227, 5R01 CA234715, 1R01 CA266452, R37 CA214955-01-A1, T32 CA009676; Department of Defense (W81XWH-20-1-0405, W81XWH-21-1-0539); Prostate Cancer Foundation Challenge Award and Young Investigator Award; the Institute for Prostate Cancer Research (IPCR); Smith Family Scholar Award; Sheppard Family Foundation Sheppard Scholar Award.
Disclosures: J. Alumkal reports consulting and speaker’s fees from Astellas Pharma, consulting fees from Dendreon, consulting fees from Merck, consulting fees from Bristol Myers Squibb, and research support to his institution from Astellas Pharma, Zenith Epigenetics, and Beactica. A. Rao reports serving as a member for Voxel Analytics, LLC. P. Nelson reports consulting fees from Merck and Bristol Myers Squibb and research support from Janssen.
Paper cited: “LSD1 promotes prostate cancer cell reprogramming by repressing TP53 signaling independently of its demethylase function,” JCI Insight. DOI: 10.1172/jci.insight.167440

Explore a variety of health care news & stories by visiting the Health Lab home page for more articles.

Department of Communication at Michigan Medicine
Want top health & research news weekly? Sign up for Health Lab’s newsletters today!